What are nuclei?
- What are nuclei?
- A nucleus is an aggregation of protons and neutrons. Protons and neutrons attract each other by exchanging particles named mesons. Further decomposing these particles, protons, neutrons, and mesons are all made of quarks joined together by exchanging gluons.
- How were nuclei created?
- When the universe, which was born by Big Bang with ultra-high temperature, went cold while expanding, protons and neutrons were created. Proton is a nucleus of hydrogen, therefore, it was the creation of hydrogen. In principle, if you combine protons and neutrons, you can make nuclei of various element. However, at this beginning stage, the universe could barely create helium, thus, only very light nuclei existed. About three hundred million years later, stars were formed from hydrogen and helium. Inside the stars, nuclear reactions have initiated and heavier elements with higher atomic number were produced. In addition to this, when stars end their life with final explosions and/or when they collide with each other, heavy nuclei are also produced while destroying some other nuclei. In this way, nucleosynthesis repeats in the universe.
See also “What is the s-process? What is the r-process?”
- How many nuclei exist?
- There are about 300 nuclides in nature, called stable nuclei. But these are just a tiny portion of nuclei. We do not know exactly how many unstable nuclei we have, which decay in short periods of time. Probably there are around 10,000 species of unstable nuclei.
See also “What are stable/unstable nuclei?”, “How does nucleus decay?” “Are there any nuclei unknown?”
- Why are there so many nuclides?
- Because nuclei are made of combination of protons and neutrons. Element species are determined solely by the proton numbers of the nuclei. Therefore, there are many different nuclei corresponding to one element.
See also “What are isotopes?”
- What are isotopes?
- Isotopes are variants of an element, with nuclei having same number of protons but different numbers of neutrons.
See also “Why are there so many nuclei?” “How should we read the notation of nuclei?”
- How should we read the notation of nuclei?
- It is customary to add the mass number at the upper left of the element symbol and to call “element name” plus “mass number”. For example, 12C is called “Carbon-Twelve”. The atomic number, which equals number of protons, is 6, so that the number of neutrons is 6, subtracting the number of protons from the mass number. Sometimes, we add the number of protons at the lower left and the number of neutrons at the lower right side of element symbol, as 126C6.
See also “What is isotope?”.
Size and weight of nuclei
- How large is a nucleus?
- The size of the smallest nucleus, hydrogen, is about 1/100,000 of the atom. Even in the case of large nuclei, as uranium, the size is about 1/10,000. To compare them by volume, the nuclear volume is 1/1,000,000,000,000 of the atomic one, or less. You cannot see it at all even with an electronic microscope. We need a special equipment with accelerators to “see” it.
- How large can a nucleus be?
- The volume of a nucleus is approximately proportional to the mass number which is the sum of the numbers of protons and neutrons. Because protons have positive charge, increasing protons and neutrons simultaneously, eventually, the electric repulsion (Coulomb interaction) between protons becomes strong enough to break it apart. Therefore, the largest nucleus existing in nature is uranium (atomic number 92). For unstable nuclei, larger nuclei can exist. For example, nihonium (Nh), produced in RIKEN, has the number of protons 113, and the number of neutrons 165. Furthermore, in the universe, there are huge (macroscopic) nuclei hold together by very strong gravity, called as neutron stars. The neutron ratio in the star is extremely high, in order to avoid the electric repulsion. This is the origin of its name.
See also “How heavy can a nucleus be?”.
- How heavy can a nucleus be?
- The mass of a nucleus is proportional to the mass number (sum of proton and neutron numbers). Besides, the volume is also proportional to the mass number, so the answer for the questions, “How large nuclei can be?” and “How heavy nuclei can be?” is same.
See also “How large can a nucleus be?”.
- Is a nucleus heavy or light?
- If there was a nucleus large enough to be visible, it would be incredibly heavy material. However, the material like this exists only under extreme environments, such as neutron stars. In fact, it is very important for us that nuclei around us have the limit for the size (weight). If there was no limit, our universe could contain only neutron stars and black halls, and human beings could not exist.
See also “Does the weight of material equal the weight of nuclei”.
- Does the weight of material equal the weight of nuclei?
- That is right. Because electrons are much lighter than nuclei, the weight of a material is almost identical to the weight of nuclei. In the other words, the weight we feel daily, including our body weight, is the weight of nuclei in the materials.
See also “Is a nucleus heavy or light?”.
Nuclei we do not know yet
- Are there nuclei unknown?
- The number of the nuclei which have been found by experiments is around 3,000. We expect unknown nuclei the same or even more than the ones discovered so far.
See also ”How many nuclei are there?”.
- How do you find nuclei unknown?
- There should exist many unknown nuclei in the universe where the nucleosynthesis is taking place. However, there is very little possibility to find them on Earth. Therefore, we generate them artificially by using accelerators. For example, at RI Beam Factory in RIKEN, they generate the unknown nuclei by colliding two nuclei either to decompose or to fuse them.
- How do you study properties of the unknown nuclei?
- If a moving nucleus goes through a magnetic field, the force proportional to its electric charge acts on it. Therefore, the nucleus changes its direction of travel according to the number of protons: With more protons, more the path bends. On the other hand, if the nuclei have more neutrons, the nucleus becomes heavier and the bending angle becomes smaller. Utilizing these characteristics, we may determine the species of the nucleus. Because the unstable nucleus decays in a short term, it is required to quickly perform experiments to study their properties.
Stable and unstable nuclei
- What are stable/unstable nuclei?
- Naively speaking, nuclei that decay into other kinds in finite time are “stable nuclei”, while those not changing are “stable nuclei”. However, the words “not changing” are somewhat ambiguous. In fact, there is no clear boundary between these two. For instance, the lifetime of uranium (238U) is about 4.5 billion years which is roughly the same as the age of the Earth. Plenty of nuclides with even larger lifetimes exist, though the actual observation of their decay is enormously difficult because it is so rare. Some exceptional measurements are bismuth (209Bi) with 1019 years, and tellurium isotopes with 1020~1024 years. These lifetimes are much larger than the age of the universe, and we may categorize them as stable nuclei.
See also “How many nuclei exist?”, “How nuclei decay?”, “What is the Heisenberg’s valley?”, “What are the neutron and proton drip lines?”
- What are ground and excited states?
- The ground state is defined as the state having the lowest energy in a given nucleus. The excited states are states with energy higher than the ground state, but they are meta-stable states that decay into lower-energy states emitting lights (gamma rays), for instance.
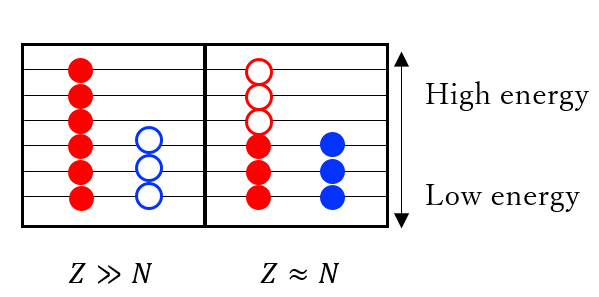
- Why do nuclei favor the same numbers of neutrons and protons?
- Mainly two reasons: (1) The attractive force between a proton and a neutron is stronger than that between two protons (neutrons). According to the Yukawa’s theory that the nuclear force is produced by the exchange of pi mesons between nucleons, this difference comes from the fact that the exchange of charged pions are allowed only between a proton and a neutron. (2) Both protons and neutrons are fermions. For the same kind of fermions, no more than one particle can sit on the single state, which is called “Pauli’s exclusion principle”. Let us take two nuclei with same mass number but different numbers of protons (See figure). We put the particles on states from the lower energy. Then, we may see that the total energy is the smallest when we have the same number of protons and neutrons.
- How does nucleus decay?
- Stable nuclei do not decay, and we need a large amount of energy to force them to decay/change. However, unstable nuclei spontaneously change themselves into more stable nuclei. There are three kinds of nuclear decay modes; alpha, beta and gamma decay.
See also “What is alpha decay?”, “What is beta decay?”, and “What is gamma decay?”
- What is alpha decay?
- This is a phenomenon to emit a nucleus of helium (two protons and two neutrons) from a heavy nucleus. After the alpha decay, the nucleus loses the mass number by four and the atomic number by two. The emitted helium nucleus is called “alpha ray”.
See also “How does nucleus decay?”, “What is beta decay?”, and “What is gamma decay?”
- What is beta decay?
- This is a reaction that a neutron in the nucleus changes into a proton, by emitting an electron and an anti-neutrino. Some nuclei show the opposite change, from a proton to a neutron. After the beta decay, the proton number changes by ±1, thus, the atomic number changes by ±1. The emitted electrons are called “beta ray”. Since the proton and the neutron approximately have the identical mass, the change in the mass is small but the nucleus becomes slightly lighter.
See also “How does nucleus decay?”, “What is alpha decay?”, and “What is gamma decay?”
- What is gamma decay?
- When the nucleus is excited (not in the ground state), it emits lights and its energy is lowered toward the ground state. These lights from the nucleus have energy larger than the X ray, and are called “gamma ray”. The gamma decay does not alter the nuclide. The gamma rays observed in nature are often associated with the beta decay. The nucleus is often excited after the beta decay, and emits the gamma ray later in transition to the lower-energy states.
See also “How does nucleus decay?”, “What is alpha decay?”, and “What is beta decay?”
- What is magic number?
- Nuclei with specific numbers of protons and/or neutrons are more stable than the surrounding nuclides. These numbers are called “magic numbers”. In stable nuclei, we know the magic numbers of 2, 8, 20, 28, 50, 82, 126. For instance, the calcium has the proton number 20 which corresponds to the magic number. Especially, 40C and 48Ca have the magic neutron numbers 20 and 28, respectively, which become even more stable as the doubly magic nuclei.
- What is the Heisenberg’s valley?
- Making the three-dimensional nuclear chart by adopting the nuclear mass as the additional vertical axis, a group of stable nuclei look like a river running in the bottom of a valley. This is called “Heisenberg’s valley”.
See also “What are stable/unstable nuclei?”
Courtesy of RIKEN Nishina Center.
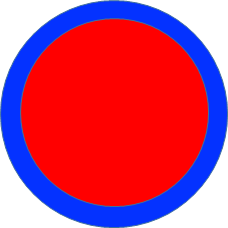
- What is neutron skin?
- In nuclei with excess neutrons, a “skin” of neutrons may be formed on the surface of the nucleus. The schematic image is shown in the figure. The distribution of neutrons shown by blue are extended outside of protons (red). The pure neutron layer (skin) exists near the surface.
- What is neutron halo?
- In nuclei with extreme number of neutrons, the energy necessary to remove a neutron from the nucleus (neutron separation energy) almost vanishes. Then, due to the quantum effect, the neutrons can be located far outside of the nucleus (with some probability). This forms a halo-like extended structure of dilute neutrons, called “neutron halo”.
- What is neutron/proton dripline?
- Fixing the proton number, the neutron separation energy decreases as the neutron number increases. We may think of the line connecting the nuclides with vanishing neutron separation energy. This is the “neutron dripline”. No more neutrons can be bound together beyond this limit which corresponds to the right boundary limit of existence on the nuclear chart. Conversely, as decreasing the neutron numbers, the proton separation energy decreases. There is another limit to bind a proton, which corresponds to the left limit of existence in the nuclear chart and is called “proton dripline”. The nuclei with large number of protons tend to be unstable due to the repulsive Coulomb interaction. Thus, the neutron dripline is located much further away from the stable line (Heisenberg’s valley) than the proton dripline. Most unknown nuclides are these neutron-rich nuclei.
See also “What are stable/unstable nuclei?”
- What is island of stability?
- Superheavy nuclei, such as Nihonium, have very short lifetimes and quickly decay after their production in the laboratory. Theoretically, we expect existence of meta-stable superheavy nuclei with significantly long lifetimes, that have much larger number of neutrons than the currently accessible superheavy nuclei. This is because the theory predicts a new magic number of neutrons to give additional stability to these nuclei. This region of meta-stable superheavy nuclei is surrounded by the sea of unstable nuclei, thus, called “island of stability”.
Shapes of nuclei
- What kind of shapes do nuclei have?
- In addition to the spherical shape, there are shapes of “lemon”-like (prolate), “pancake”-like (oblate), and “kiwi”-like (triaxial). These shapes have a common feature that the cut section becomes elliptic. There are also nuclei showing pear shapes (octupole). In excited states, we expect even more exotic shapes, such as tetrahedron, banana, and rod-like shapes. Furthermore, in the universe, theories predict a variety of nuclear shapes near the surface of neutron stars; rod-like, slab-like, hole-like, etc.
- Does the deuteron look like a dumbbell?
- The deuteron (nucleus of deuterium) consists of a proton and a neutron. However, it is not like a dumbbell shape of diatomic molecules, because the distance between the proton and the neutron is not fixed because of large quantum fluctuation. This quantum nature is one of important aspects of nuclei. It is mysterious and interesting that such systems with strong quantum nature show various shapes.
- What are the deformation parameters?
- They indicate how far the shape is deviated from the sphericity. There are several kinds of deformation parameters. In “Interactive Nuclear Chart”, we show beta, gamma, and octupole deformation parameters. The octupole deformation parameter indicates degree of the pear shape. The beta deformation indicates degree of deviation from the spherical shape, and the gamma parameter determines the type of deformation. The gamma parameter γ=0 corresponds to a simple stretch of the spherical shape (lemon-like), γ=60° corresponds to a squashed shape (pancake-like). In between 0 and 60°, the lemon-like shape is squashed from the side (kiwi-like).
- Why does the shape vary from nucleus to nucleus?
- The deformation away from the spherical shape is a consequence of phenomena called “spontaneous breaking of symmetry”. We can predict nuclear shapes from numerical calculations, however, it is difficult to answer why it has such a shape. We know the shell effect, which is peculiar to the quantum mechanics, plays a crucial role to determine the shape, but cannot easily explain why such shapes are formed. Go to “Interactive Nuclear Chart” and look at shapes of many nuclei. You can find spherical nuclei near the magic numbers. As increasing the proton/neutron number, it changes into lemon-like shapes and increases magnitude of deformation. Further increasing the proton/neutron number and approaching to the next magic number, their shapes changes into kiwi-like, then, pancake-like shapes, before going back to the spherical shape. There are some exceptions, but roughly speaking, this is how the shape changes on the nuclear chart.
- What happens when the nuclear shape changes?
- First of all, we find big changes in excited states in the nucleus. In the quantum systems, a spherical system cannot rotate, while a deformed one can do. Therefore, the characteristic excitation spectra, called “rotational band”, appear in excited states. If the nucleus is deformed into the pear-like shape, another kind of characteristic excited states (parity doublet) appear. The deformation also affects ground-state properties of nucleus, such as radius and lifetime.
- What kind of simulation is performed to find nuclear shapes?
- We are using the density functional theory for calculations of nuclear shapes shown in “Interactive Nuclear Chart”. This theory is based on the density functional which defines the energy in terms of density distribution of protons and neutrons. We determine their density distribution by minimizing the energy. In practice, this minimization is done by iteratively solving wave equations similar to the Schrodinger equation in the quantum mechanics.
- How long is the computation time to find nuclear shapes?
- It varies very much, depending on the adopted assumption, restriction, approximation, etc. The calculation we have done for “Interactive Nuclear Chart” takes a few to ten hours for each nucleus, using an average PC.
To produce and transform nuclei
- What is the s-process? What is the r-process?
- Both of them are processes in the universe to produce elements (nuclei) heavier than the iron. The s-process (“slow process”) is a chain of alternating repetition of the neutron absorption and the beta decay, to produce heavy nuclei in giant stars. This process proceeds very slowly, thus, it goes through the stable nuclei. On the other hand, the r-process (“rapid process”) occurs under environments with enormous number of neutrons available. The nucleus absorbs many neutrons faster than their beta decay, producing heavy neutron-rich nuclei. After the nuclear reaction stops, the neutron-rich nuclei changes neutrons to protons by successive beta decay until they reach the stable nuclei. The r-process must take place somewhere in the universe, however, we still do not know where and how it does. We used to think that the supernova was the r-process site. Recently, other explosive stellar events, such as neutron-star merger, become the candidates too. See also “How did nuclei begin?”
- Can we artificially create and transform nuclei?
- We cannot create nuclei from nothing, but we can transform a nucleus to different nucleus. This is called “nuclear transmutation”. Rutherford, who discovered the nucleus, did it for the first time in the history, creating oxygen from nitrogen bombarded with alpha ray.
- Can we change the lifetime of nuclei?
- Transforming it into other nuclide, the lifetime could be either longer or shorter. Since the energy scale of nucleus is very large, the lifetime hardly changes, even if the radioactive element is embedded in the material. There are nuclei that change their lifetimes by stripping all electrons around the nucleus. See also “Can we artificially create and transform nuclei?”.
- Can we deactivate radioactive elements?
- We can do this by transforming unstable nuclei into stable ones. However, we have not achieved yet to deactivate radioactive materials by massive transmutation of radioactive elements only. It is still difficult with the present human technologies to yield massive and specific nuclear reactions.
See also “Can we artificially create and transform nuclei?”.